Pharma Outcomes That Matter and How Intelex Helps Deliver Them
8 minute read In pharmaceutical manufacturing, the difference between a routine audit and a serious regulatory escalation often depends on the evidence you can provide. That evidence needs to clearly show that your data is consistent across all systems,
Latest Posts
- Health & Safety Management
Where Pharma Compliance Quietly Breaks
6 minute read Pharma EHS and Quality teams manage compliance as part of their daily work. The pressure surfaces when an audit begins, and routine questions suddenly require days of coordination to answer. In these moments, the challenge is rarely a lack of...
- Software Features and Tutorials
Safety Classification and Learning Model: The Framework for Consistent Learning
4 minute read When an incident occurs, how do you classify it? Is it a serious injury and fatality (SIF)? A potential SIF? When people answer that question differently, it becomes a data quality and a resource allocation nightmare. Research conducted by the...
- Software Features and Tutorials
Energy-Based Observations: Identify Critical Hazards and Verify Controls
5 minute read If you ask a safety professional about their observation data, you’ll often hear the same thing: there are plenty of observations, but it’s hard to know what to do with the information. Most of the information from observation,...
- Intelex News and Updates
Q1 2026 Product Release Notes: New AI Workflows, ACTS Updates, and More
Intelex’s Q1 2026 product update focuses on increasing efficiency, accelerating time to value, and empowering...
- Software Features and Tutorials
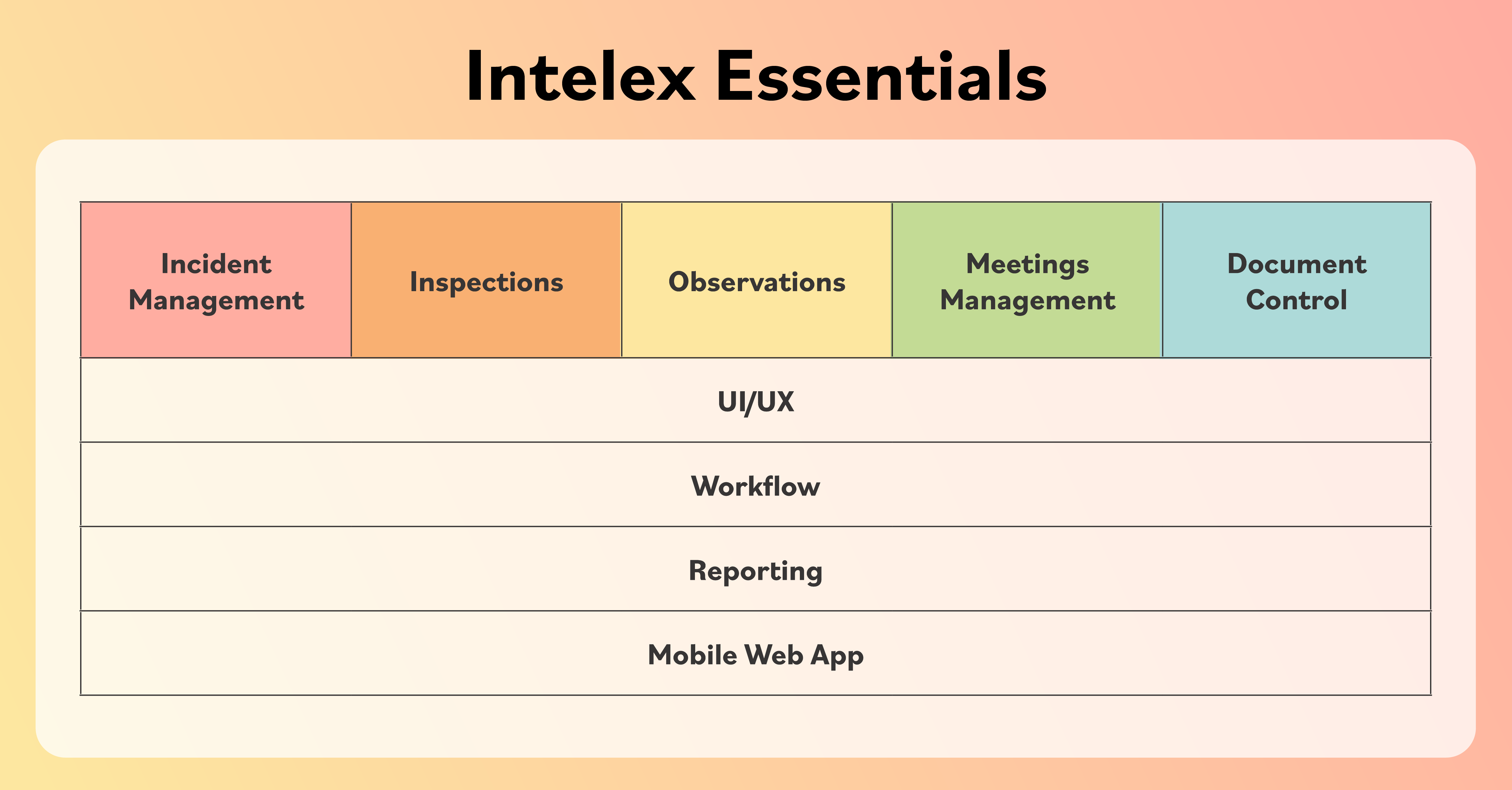
Roll Out EHS Software Faster with Intelex Essentials’ DIY Implementation Manager
See how non-technical EHS managers can set up professional safety software on their own in 4-6 weeks with Intelex...
- Software Features and Tutorials
Energy-Based Safety: A New Approach to Preventing Serious Injuries and Fatalities
5 minute read Here’s an uncomfortable truth: while Total Recordable Injury Rates have dramatically reduced over the last 20 to 30 years, the fatality rate has stayed flat. What this tells us is that we’ve gotten good at preventing relatively minor...